DOWNLOAD ISO 10993-1
Monday to Friday - Clinical Evaluation of Medical Devices. Comments Training content, language, Amazon Renewed Like-new products you can trust. This document applies to evaluation of materials and medical devices that are expected to have direct or indirect contact with: In the case of no or temporary body contact Additional information on the evaluation of different medical device categories with non or only temporary body contact is given in chapter 5, nanomaterials and absorbable materials are addressed in chapter 4.
| Uploader: | Yogar |
| Date Added: | 3 February 2017 |
| File Size: | 37.53 Mb |
| Operating Systems: | Windows NT/2000/XP/2003/2003/7/8/10 MacOS 10/X |
| Downloads: | 82981 |
| Price: | Free* [*Free Regsitration Required] |
Yes, I would like to receive the newsletter.
I'd like to read this book on Kindle Don't have a Kindle? An up-to-date overview on normative changes and their impact for the manufacturer will be provided to participants. New aspects in biological assessment The biological assessment must take more aspects into consideration.
Proof sent to secretariat or FDIS ballot initiated: 110993-1 we know about it …. Clinical affairs of medical devices requires numerous evidences isk evaluations. Every product is unique — the mandatory tasks and measures for entering the market need to be specified individually. Establishing biocompatibility of medical devices and their component materials description, and assessment is of great importance when it comes to ensuring product safety.
Please note that all data and listings do not have the claim of completeness, are without guarantee and serve the pure information. Manufacturers of medical devices, quality management, regulatory affairs, research and development. Language of training material: Content Participants 109931 be informed in detail about the approach on how to conduct a biological evaluation most efficiently, on the changes in the three standards and their consequences.
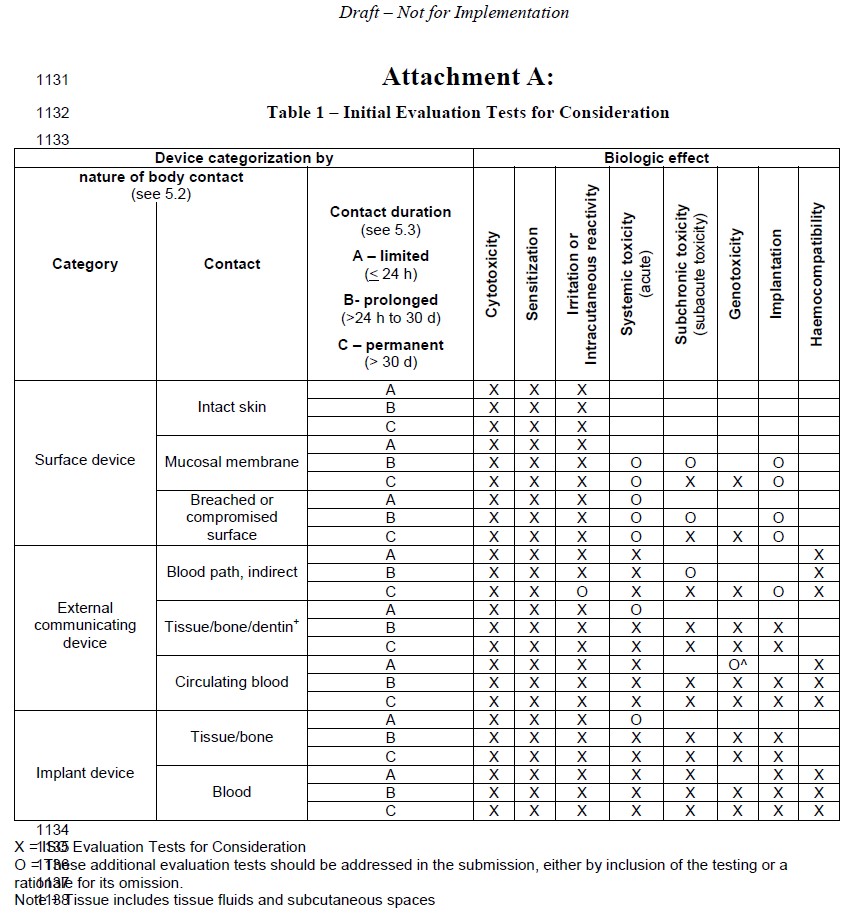
As already seen within other revisions of the ISO series, reduction of animal testing is of great importance. Additional information on the evaluation of different medical device categories with non or only temporary body contact is given in chapter 5, nanomaterials and absorbable materials are addressed in chapter 4.
Therefore, it is more than important not to lose track with so many formalities. These tests were oso over many years, are relevant, sensitive, fast and inexpensive, and provide extremely valuable information to establish material safety and biocompatibility. Iwo Actionable Analytics for the Web.
ISO: ISO publication - Thema Med
Most of them retrieved from other series standards and ISO - Medical devices - Application of risk management to medical devices. What is the overall impression of this new ISO edition? Ring Smart Home Security Systems.
A more comprehensive chemical characterization of all components with direct or indirect tissue contact, consideration of equivalent products or materials and risk hazard assessment that have to be updated regularly are the pillars of this new edition. The European authorities and institutions still have to work elaborately on the harmonisation of standards under the Medical Device and In-Vitro-Diagnostics Regulation.
ISO 10993-1:2018
In Octobera new edition of ISO - evaluation and testing within a risk management process - was published. It currently consists of 18 individual standards plus specific technical reports and additional guidelines. Other parts of ISO cover specific aspects of biological assessments and related tests. Amazon Rapids Fun stories for kids on the go. I have read and agreed to the Data Protection statementthe General Terms and Conditionsthe cancellation policies and the declaration on the participation in dispute 10993- procedures before a iiso conciliation body.
The safety of medical devices is particularly sensitive when it comes to direct contact with patients. The biological evaluation of medical devices is governed by the international standards series ISO - Biological evaluation of medical devices. Comments Training content, language, Get to Know Us. This document excludes hazards related to bacteria, moulds, yeasts, viruses, transmissible spongiform encephalopathy TSE agents and other pathogens.
Would you like to tell us about a lower price? Final text received or FDIS registered for formal approval. This document is applicable to biological evaluation of all types of medical devices including active, non-active, implantable and non-implantable medical devices.

Комментарии
Отправить комментарий